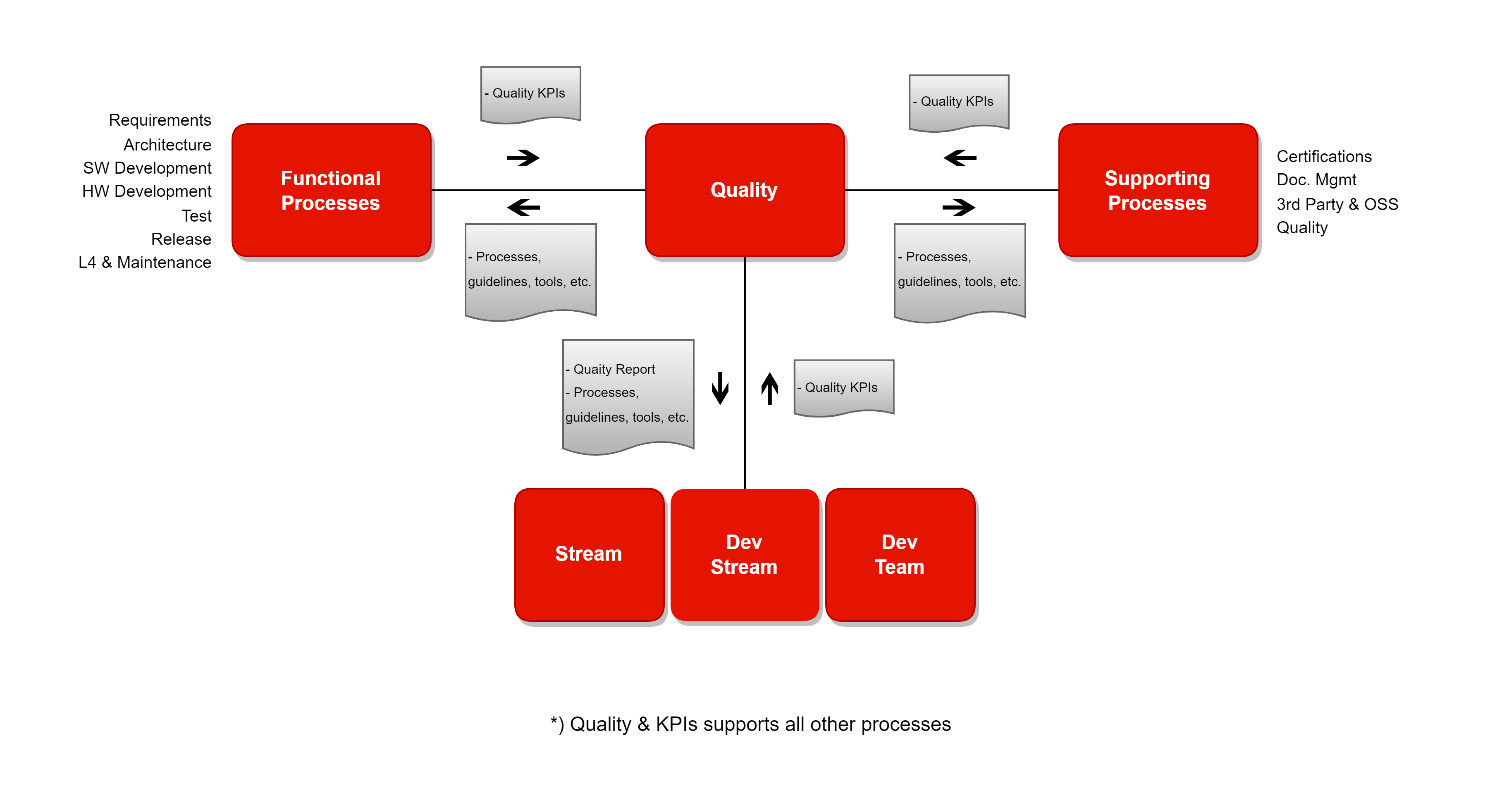
Quality & KPIs
The quality process describes how to create and maintain the PCP R&D processes. It also includes quality control, definition and evaluation of KPIs, and how to facilitate process improvements.
The main PCP R&D quality objectives are:
- Build trust in products and services provided to divisions and customers, and make ABB a partner in enabling an efficient, reliable, and future-proof plant operation.
- Achieve and sustain a good quality of products and services. PCP R&D uses processes with clear definitions of roles and responsibilities.
- Collecting and analyzing data from the processes. Dashboards are accessible to stakeholders for transparency and enable fact-based decision-making.
note
This process covers R&D quality. It focuses on how the R&D processes are managed, applied, and followed up in streams/releases (quality control). The quality checks of deliverables are built-in and checked in other processes.
Examples of quality-related activities:
- SW development (design reviews, code reviews, static code analysis, and unit tests)
- Test (different test activities to ensure the quality)
- Release process (all deliverables completed for the release)
- Quality process ("Definition of Ready" and "Definition of Done")
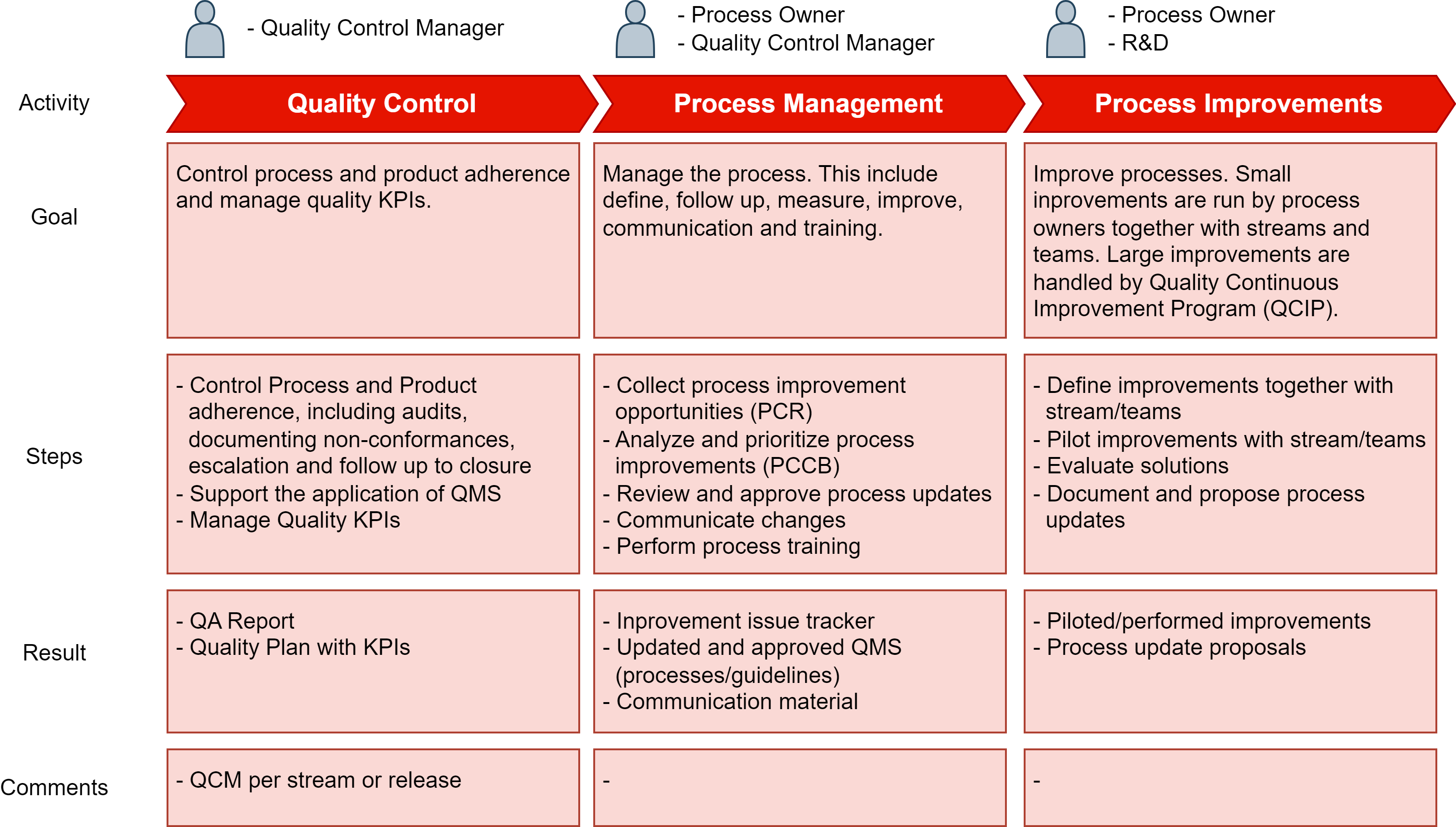
Process Overview
Principles
- Quality Control (QC)
- Regularly assess adherence to processes and the quality of work products, and follow up deviations to closure.
- Support the application of the Quality Management System.
- Define, monitor, and assess quality KPIs (for streams/releases) and escalate severe quality issues to management.
- Process Management
- R&D processes shall be created, measured (KPIs), controlled, and maintained according to the governance structure.
- Communicate changes to the QMS to management and practitioners.
- Collect process training needs as a basis for regular process training sessions
- Process Improvements.
- Deficiencies identified, analyzed, and resolved to manage quality issues in systems and products.
- Process improvements to refine and improve existing QMS processes and tools. Alternatively changes to include new features and functionality or replace existing functionality in the QMS.
Activities
Artifacts
| Artifact | Description | RACI | Receiver | Comments |
|---|---|---|---|---|
| Quality Report | Plans and status of the process and product adherence, and quality KPIs | (R): Quality Control Manager (A): Head of Quality Control (C): Release Owner (I): Configuration Manager | Stream Owner | Can reference to information in DevOps |
| Quality Plan 1) | Describes how the QMS is applied with tailoring, quality KPIs, quality control and quality assurance. | (R): Release Owner (A): Stream Owner or Functional Safety Management Manager1) (C): Quality Control Manager, Safety Engineer1) (I): Configuration Manager, Development Team, Test Lead | Stream Owner | 1) For safety-related projects, the Functional Safety Management (FSM) Manager is accountable (A) instead of the Stream Owner, and the Safety Engineer (SE) is also consulted (C) along with the Quality Control Manager (QCM). |
| Quality Dashboard(s) | Dashboards in Azure DevOps visualizing quality KPIs. | (R) Release Owner (A): Stream Owner (C): Quality Control Manager (I): SteCo | Stream Owner | - |
| Process Change Request (PCR) | Process improvement or change ticket in Azure DevOps | (R) Process Team Member (A): Process Owner2) (C): Experts in PCP R&D3) (I): (*) | R&D | 2) PRO can delegate accountability to a Process Team Member who can close the PCR. The Process Team Member cannot be the same as the responsible, (R), for the PCR. 3)The consulted “Experts in PCP R&D” are defined with respect to the content of the PCR. This role can be assessed as not relevant depending on the content of the PCR |
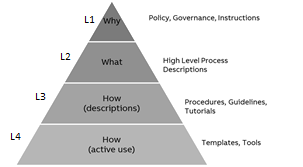
| QMS - L1 | Policy/governance - set of goals, policies, decision-making, and organization. | (R) Head of Quality (A): Head of R&D (C): Head of OpEx (I): Process Owner4), OpEx, (*) | R&D | 4) Only relevant PROs to the review/pull request |
| QMS - L2 | Processes – high-level description of activities, roles, and artifacts to achieve a particular goal | (R) Process Team Member (A): Process Owner (C): Experts in PCP R&D3) (I): Process Owner4), OpEx, (*) | R&D | 3)The consulted “Experts in PCP R&D” are defined with respect to the content of the PCR. This role can be assessed as not relevant depending on the content of the PCR 4)Only relevant PROs to the review/pull request |
| QMS - L3 | Guidelines - a recommended and detailed description of how to perform an activity or task | (R) Process Team Member (A): Process Owner (C): Experts in PCP R&D3) (I): Process Owner4), OpEx, (*) | R&D | Stream Owner can establish local tailoring 3)The consulted “Experts in PCP R&D” are defined with respect to the content of the PCR. This role can be assessed as not relevant depending on the content of the PCR 4)Only relevant PROs to the review/pull request |
| QMS - L4 | Templates/instructions/tools | (R) Process Team Member (A): Process Owner (C): Experts in PCP R&D3) (I): Process Owner4), OpEx, (*) | R&D | Stream Owner can establish local tailoring 3)The consulted “Experts in PCP R&D” are defined with respect to the content of the PCR. This role can be assessed as not relevant depending on the content of the PCR 4)Only relevant PROs to the review/pull request |
The Process Owner can delegate to a process team member, but a minimum of four eyes in the review is required for approval
Dependencies
References
Related
Owner: Quality and KPI Team