How-to Create Process Change Requests
This guide takes you through the steps to initiate a change on the PCP R&D Processes website by creating a process change request (PCR). It also includes information on how the process teams handle submitted PCRs and updates to the R&D processes.
A PCR can be created for improvements, missing information, or defects found in the processes, guidelines, etc. PCRs are also used to track document/template change requests. Document updates and/or withdrawals follow the DMS process, which is outside of R&D. The process team will track the activities through the PCR which will be closed after the document approval.
Note: Before creating a new PCR, discuss your improvement idea with the process owner (PRO) or a process team member. This helps to avoid duplicated PCRs and prevents unnecessary work.
Intended for
Anyone in PCP who would like to file a change request for PCP R&D processes.
Activities
Note: Creating a PCR in Azure DevOps (ADO) requires an ADO license. If you don't have one, ask the process owner for help creating the PCR.
Improvement ideas should be carefully evaluated and planned, with attention to their impact on other process areas. Broader-scope improvement ideas should be managed as features, while individual, targeted changes can be tracked as PCRs. When multiple PCRs relate to the same area or topic, consolidating them under a single feature is highly recommended to simplify dependency management and enhance overall process coordination.
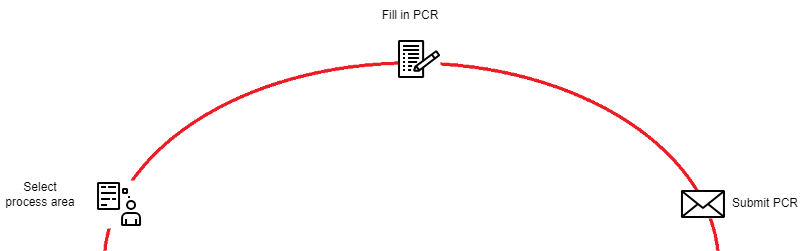
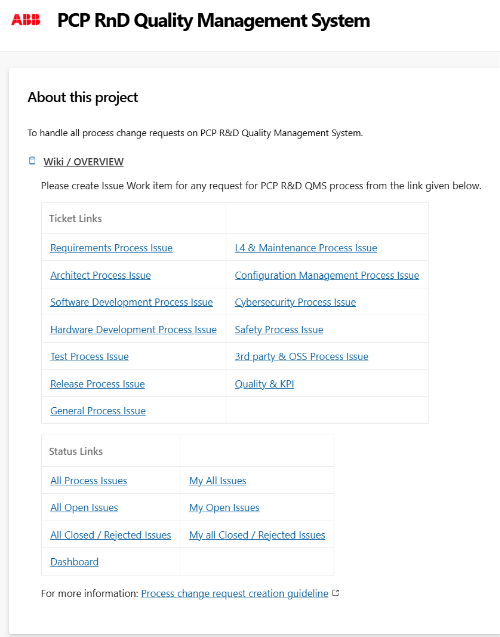
Select process area
Go to the PCP R&D PCR site and select the process area you want to create a PCR for.
Fill in PCR
The required fields in a PCR are:
- Title
A short description of the requested change. - Description
Motivate change, origin, impacted document name/ID/revision, or tool template name with the problem statement and expected change. If the PCR is for withdrawal of wiki or DMS content, ensure that reference to any replacement is included in the PCR description.- Affected standards
Specify affected standard(s) like IEC61508, IEC62443, ISO9001. - Links/attachments
Hyperlink the affected document/page location and section, or attach the proposal/screenshots and other details.
- Affected standards
- Priority
Select from 1-4 as per the definition. The priority (1=High… 4=Low) defines the order for the process team to handle the request. It can be redefined by the process team depending on capacity. - Due date
Select an "expected due date" by the originator if the priority is 1 (immediate).
Submit PCR
Click on "Save" to submit your PCR.
Details
PCR escalation
If needed, the process owner / team can escalate a PCR according to the following escalation ladder:
- PCP R&D Management
- OpEx SteCo
- PRO Sync meeting
- Process team
The reason for an escalation of PCRs can be that resources aren't available, it affects several process teams, it's not aligned with management expectations, or it has dependencies on other processes outside R&D. PCP R&D Management is the highest authority in R&D, for cross-cutting issues over many organizational functions please refer to PCP PMO or PCP BMS teams.
PCR evaluation
The process team evaluates the PCR and, if needed, contacts the originator to clarify missing information. After the evaluation by the process team, it should be clear what actions to take to resolve the PCR.
PCRs across several process teams can be discussed in PRO sync meetings. It may result in a split of the PCR for each process team.
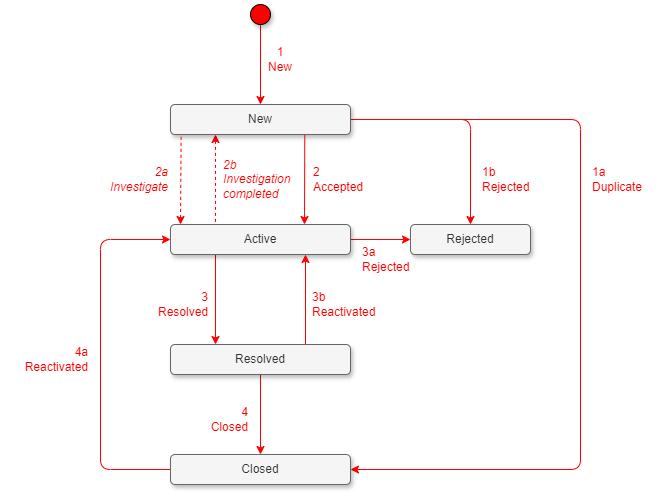
| Id | State Change | Reason | Description | Mandatory fields |
|---|---|---|---|---|
| 1 | New | Moves to state New. | A new issue is found and logged by anyone. | - Assigned to: PRO. - Title, description, hyperlinks. Set "expected Due Date" and "priority". |
| 1a, 1b, 3a | New to Closed/Rejected, Active to Rejected | Duplicate, rejected (edit reason manually). | PRO reviewed the PCR and found duplicate/invalid. | - Assigned to: blank. - Update "Process Team Feedback," edit the reason as "duplicate" or "rejected" manually, and link to other open issue work items before closing. |
| 2 | New to Active | Moved out of state "New" is considered as accepted/approved. | The PCR is approved to be corrected. | - Assigned to: process team member. - Priority, stack rank, safety impact. - Discussion; using @ to notify additional members. |
| 2a (optional) | New to Active | Investigate (edit reason manually). | The PCR is sent for investigation and assigned by the PRO to a specific process team member. | - Assigned to: process team member. Discussion; using @ to notify additional members. |
| 2b (optional) | Active to New | Investigation complete (edit reason manually). | PCR moved back to state "New" by the PRO with the reason "investigation complete" to be processed by team member. | - Assigned to: PRO. - Hyperlink to impacted processes. - Committed due date, safety impact. - Set stack rank (within the backlog). - Process team feedback (proposed solution). |
| 3 | Active to Resolved | Moved to state "Resolved" is considered as fixed/ready for pilot. | PCR implementation complete/ready for pilot/training. Communicate to the originator. | - Assigned to: process team member. - Process team feedback (final solution). - Hyperlink to changed processes. |
| 3b | Resolved to Active | Reactivated (edit reason manually). | Solution not meeting expectations of PRO. | - Assigned to: PRO. - Discussion; using @ to notify additional members. |
| 4 | Resolved to Closed | Moved out of state "Resolved," it's considered validated. | PCR completed, solution met. Documents updated, pilots/training successful: the PRO can close it. | - Assigned to: blank. |
| 4a | Closed to Active | Reactivated. | Closed by mistake. | - Assigned to: PRO. - Discussion; using @ to notify additional members. |
PCP R&D Processes website updates
The PCP R&D Processes website is made with Markdown files, versioned, and baselined. Pull requests in ADO are used to review and approve content before it is published on the web server. Content editing, reviewing, and approval are restricted to the process teams.